The Electric Force
- Kamran Jalilinia
- kamran.jalilinia@gmail.com
- 18 min
- 98 Views
- 0 Comments
Introduction
Electromagnetism is a branch of physics and engineering that includes the study of electric and magnetic fields and their interactions. Electricity and magnetism are two aspects of electromagnetism. This concept describes how electric currents create magnetic fields and how changing magnetic fields induce electric currents. Electromagnetism involves phenomena such as electromagnetic induction, electromagnetic waves (including light), and the behavior of charged particles in electric and magnetic fields.
In physics, electromagnetism is one of the fundamental forces of nature. In engineering, electromagnetism plays a crucial role in various disciplines such as electrical engineering, electronics, telecommunications, and electromechanical systems. Engineers always utilize principles of electromagnetism to design and develop devices like motors, generators, transformers, antennas, and communication systems.
Historically, electricity and magnetism were long thought to be separate forces. It was not until the 19th century that they were finally treated as interrelated phenomena. In 1905 Albert Einstein’s special theory of relativity established that both are aspects of one common phenomenon.
An important aspect of electromagnetism is the science of electricity, which is concerned with the behavior of aggregates of charge, including the distribution of charge within matter and the motion of charge from place to place.
At a microscopic scale, the electric force in particular is responsible for most of the physical and chemical properties of atoms and molecules. It is strongly compared with the force of gravity. At a more familiar macroscopic scale, electric phenomena are responsible for the lightning and thunder accompanying certain storms in nature. Such conditions can be simulated in a laboratory with two oppositely charged metal spheres which are installed on insulated stands. If we bring them close to each other, in a certain distance we may observe some sparks because of electrical discharge between spheres, as Figure 1 shows.
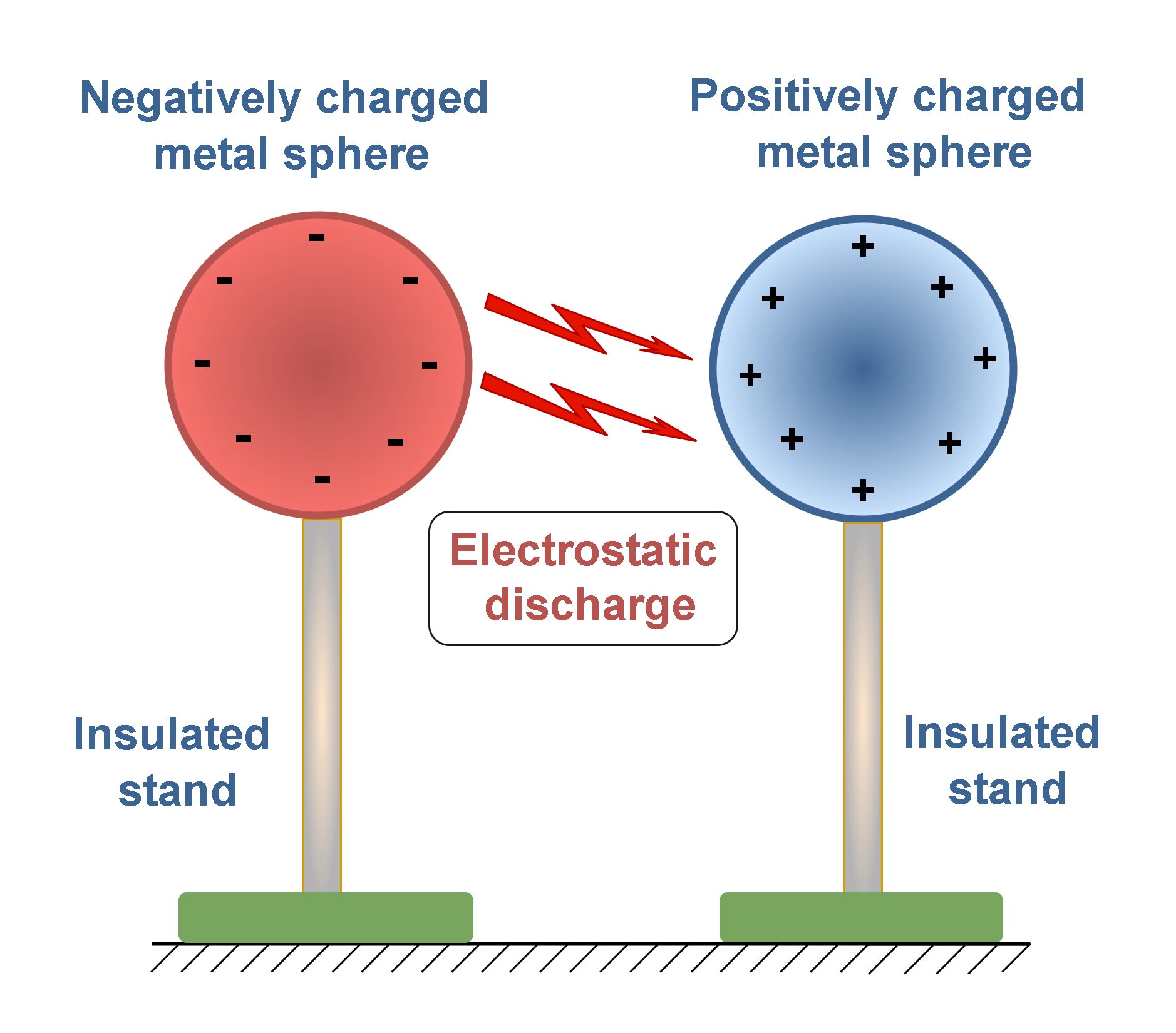
Electricity is the lifeblood of technological civilization and modern society. Without it, we revert to the mid-nineteenth century: no telephones, no television, none of the household appliances that we take for granted. Instead, with the discovery and harnessing of electric forces and fields, we can view arrangements of atoms, probe the inner workings of the cell, and send spacecraft beyond the limits of the solar system.
Static Electric Charges
Electrostatics is a branch of physics that studies the interaction between slow-moving or stationary electric charges. It focuses on phenomena involving static electricity, where charges are not in motion.
Around 600 B.C. the ancient Greek philosophers conducted the earliest known study of electricity. It all began when Thales noticed that a fossil material called amber would attract small objects after being rubbed with wool because it became electrically charged. Subsequent experiments found that most materials when rubbed possessed this property. We say that they are electrified (a word derived from elektron, the Greek name for amber).
An officer in the French Army Engineers, Colonel Charles Coulomb (1736- 1806), performed an elaborate series of experiments to determine quantitatively the force exerted between two objects, each having a static charge of electricity.
Experiments also demonstrate that there are two kinds of electric charge, which the American scientist Benjamin Franklin (1706- 1790) named positive and negative charges.
A charge is a basic property of matter. Most bulk matter has an equal amount of positive and negative charge and thus has zero net charge.
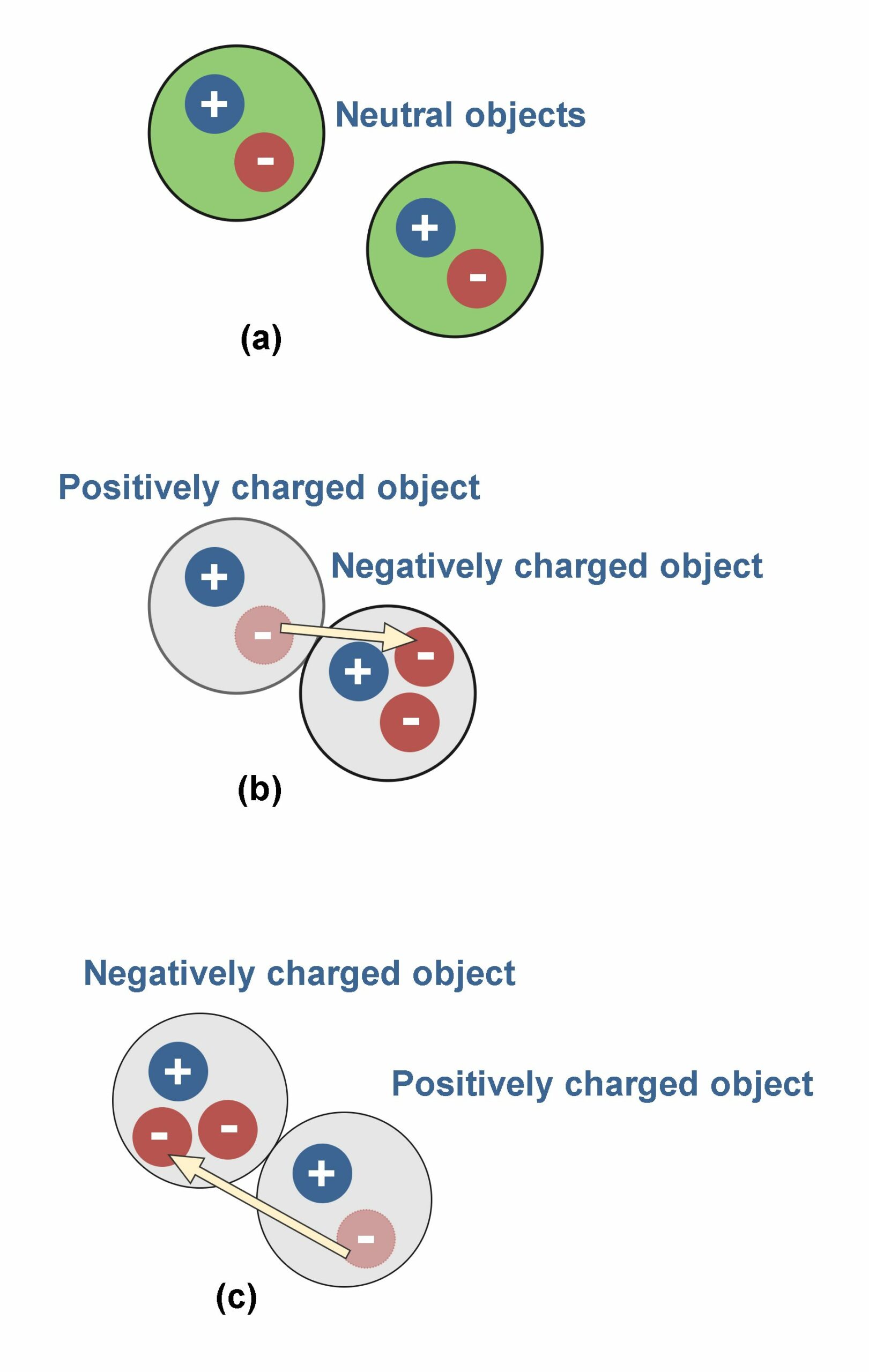
Figure 2 illustrates the interaction of two charges. There are 2 objects (for example 2 metallic spheres) suspended by insulated strings.
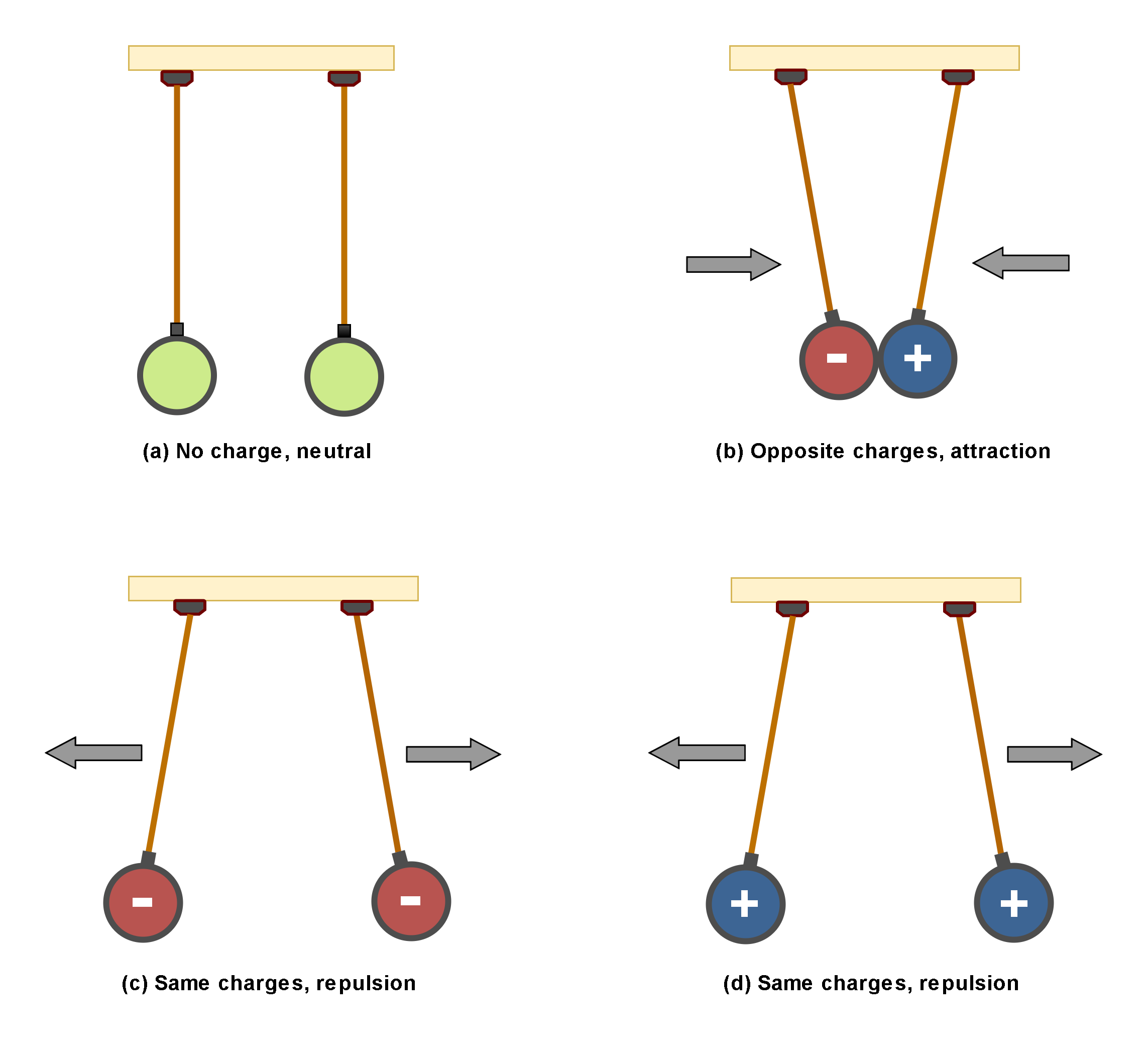
Figure 2: The interaction of two charges
In Figure 2(a), both of objects are uncharged and there is not any interaction between them. In Figure 2(b), they are oppositely charged and then there are attraction forces between them. This is one of the main physical principles that charges of opposite signs, attract each other.
In Figure 2(c) and 2(d), the objects are similarly charged and then there are repulsive forces between them. This is another physical fact that charges of the same signs, repel each other.
As a more realistic example, Figure 3 shows an experimental setup for observing the electrical force between two charged objects.
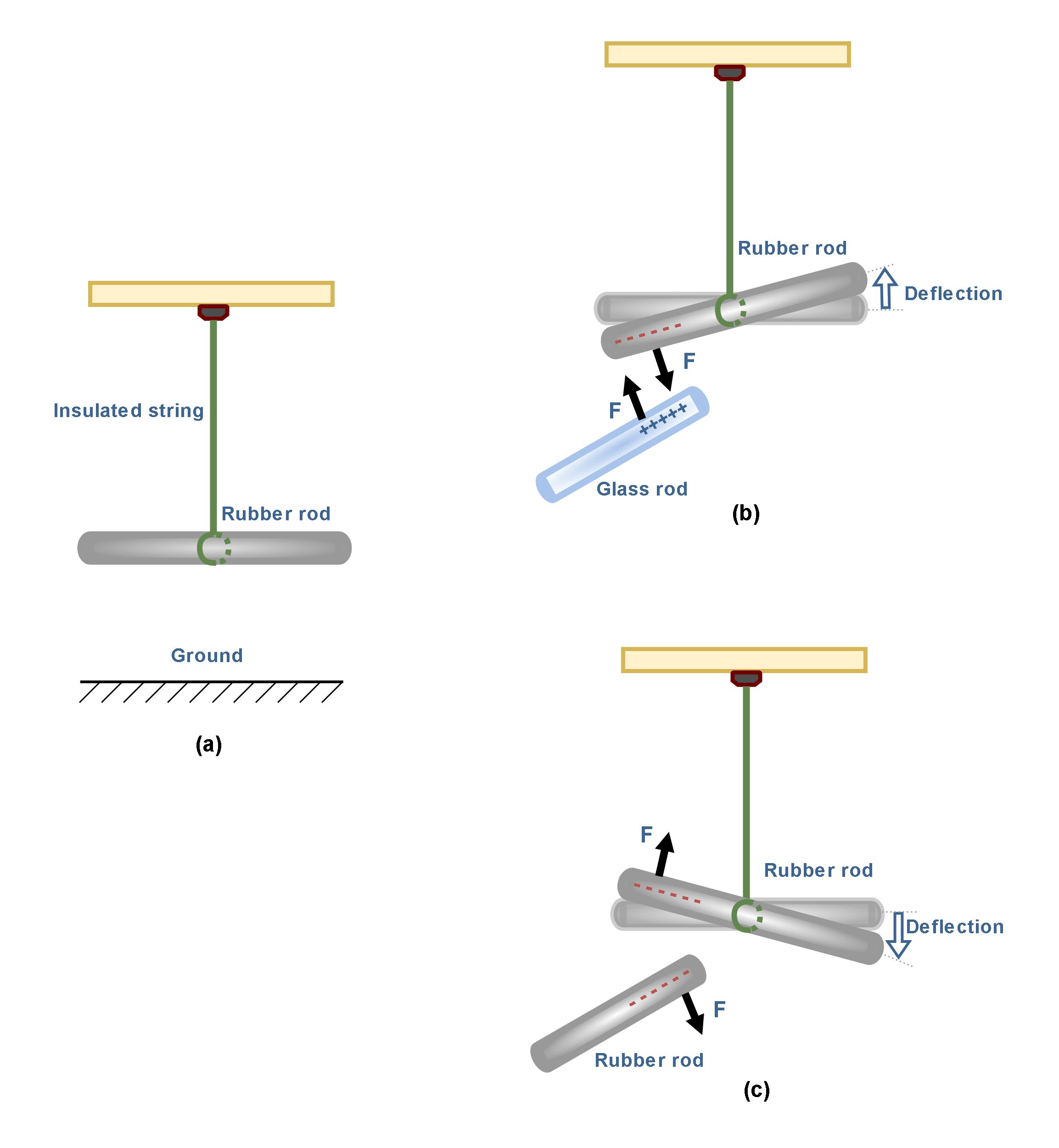
In Figure 3(a) there is an uncharged hard rubber rod that is suspended by a piece of insulated string over the ground. We might assume that the rubber rod has already been rubbed with fur before suspending. Then, it has absorbed some negative charges. Also, we have a glass rod which has already been rubbed with silk and then it has lost some negative charges. When the positively charged glass rod is brought near the rubber rod, the rubber rod is attracted toward the glass rod because the electrostatic force between them is attractive as shown in Figure 3(b).
If two charged rubber rods (or two charged glass rods) are brought near each other, as in Figure 3(c), the force between them is repulsive. These observations may be explained by assuming the rubber and glass rods have acquired different kinds of charge, where the electric charge on the glass rod is positive and that on the rubber rod is negative.
Atomic Nature Of Electricity
Atoms are the basic particles of the chemical elements. The word atom is derived from the ancient Greek word “atomos”, which means “uncuttable”.
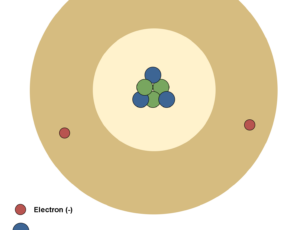
In atomic physics, the Rutherford–Bohr model of the atom, presented by Niels Bohr and Ernest Rutherford in 1913, consists of a small, dense nucleus surrounded by orbiting electrons. It is analogous to the structure of the Solar System, but with attraction provided by electrostatic force rather than gravity, and with the electron energies quantized (assuming only discrete values). Figure 4 shows the simplest classic physical model of an atom.
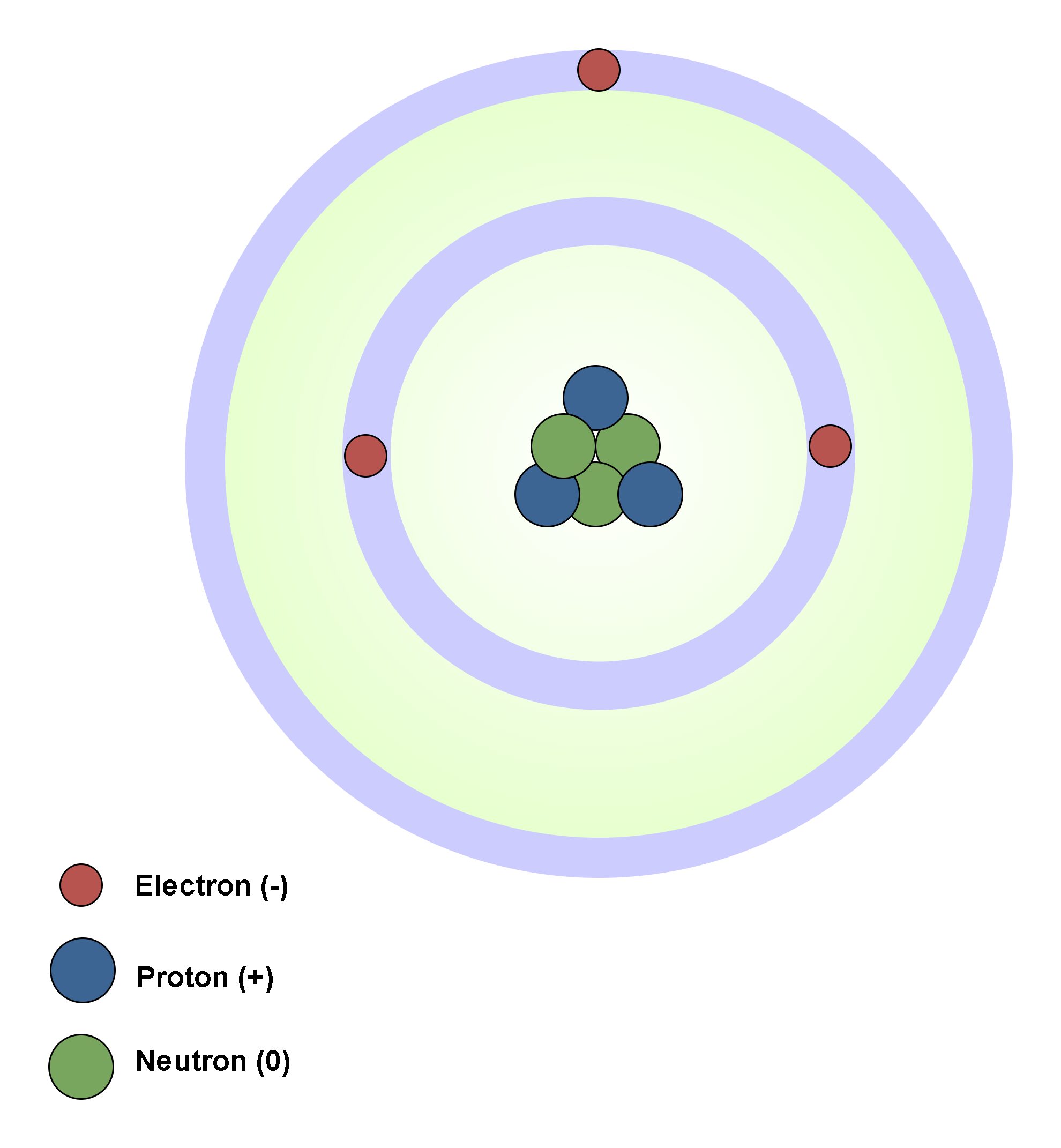
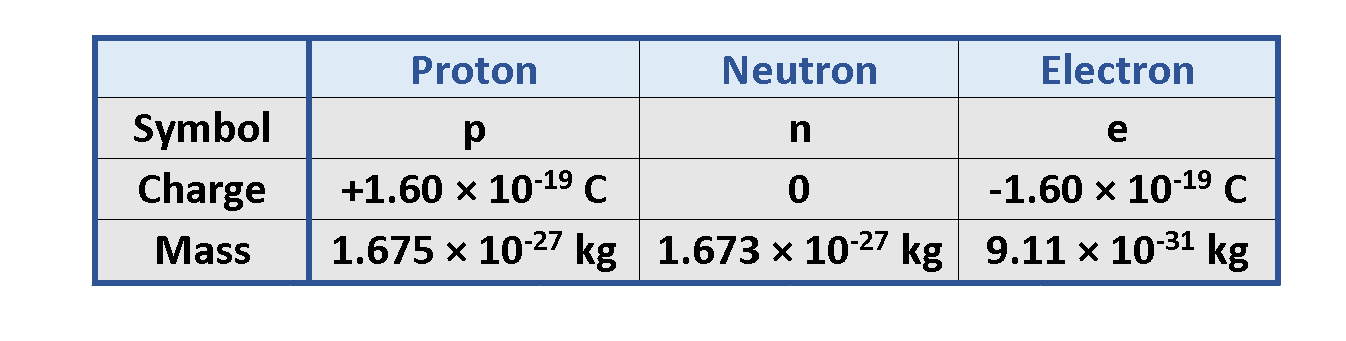
Atom´s nucleus contains practically the whole mass of the atom. It consists of protons and neutrons. Neutrons are not charged. Protons are charged positively and they never move from one material to another. Electrons are small and light negatively charged that rotate around the core in electron shells. They occupy the outer regions of the atom.
In 1909 Robert Millikan (1886–1953) discovered that if an object is charged, its charge is always a multiple of a fundamental unit of charge, designated by the symbol e. Other experiments in Millikan’s time showed that the electron has a charge of -e and the proton has an equal and opposite charge of +e. In modern terms, the charge is said to be quantized, meaning that charge occurs in discrete chunks that can’t be further subdivided.
In the SI (International System of Units) the unit of electric charge is the Coulomb or C. The amount of charge of one proton is qp = +e = 1.60 × 10-19 C.
Table 1 provides information about particles of an atom containing the charge and mass of each component.
Electrons are far lighter than protons and hence more easily accelerated by forces. A typical atom contains many electrons that can be closer to or further from the core. Those further from the core are loosely bound and they can be removed by rubbing or other methods. Rubbing the two materials together serves to increase the area of contact, facilitating the transfer process.
Normally atoms are not charged. A neutral atom (an atom with no net charge) contains as many protons as electrons. Removing electrons creates a positively charged ion and placing additional electrons on the atom creates a negatively charged ion. Consequently, objects become charged by gaining or losing electrons.
Essentially, 1C is a very large amount of charge. In typical electrostatic experiments in which a rubber or glass rod is charged by friction, there is a net charge on the order of 10-6 C.
As a tangible example, an ordinary flashlight battery delivers a current that provides a total charge flow of approximately 5,000 Coulombs, which corresponds to more than 1022 electrons, before it is exhausted!
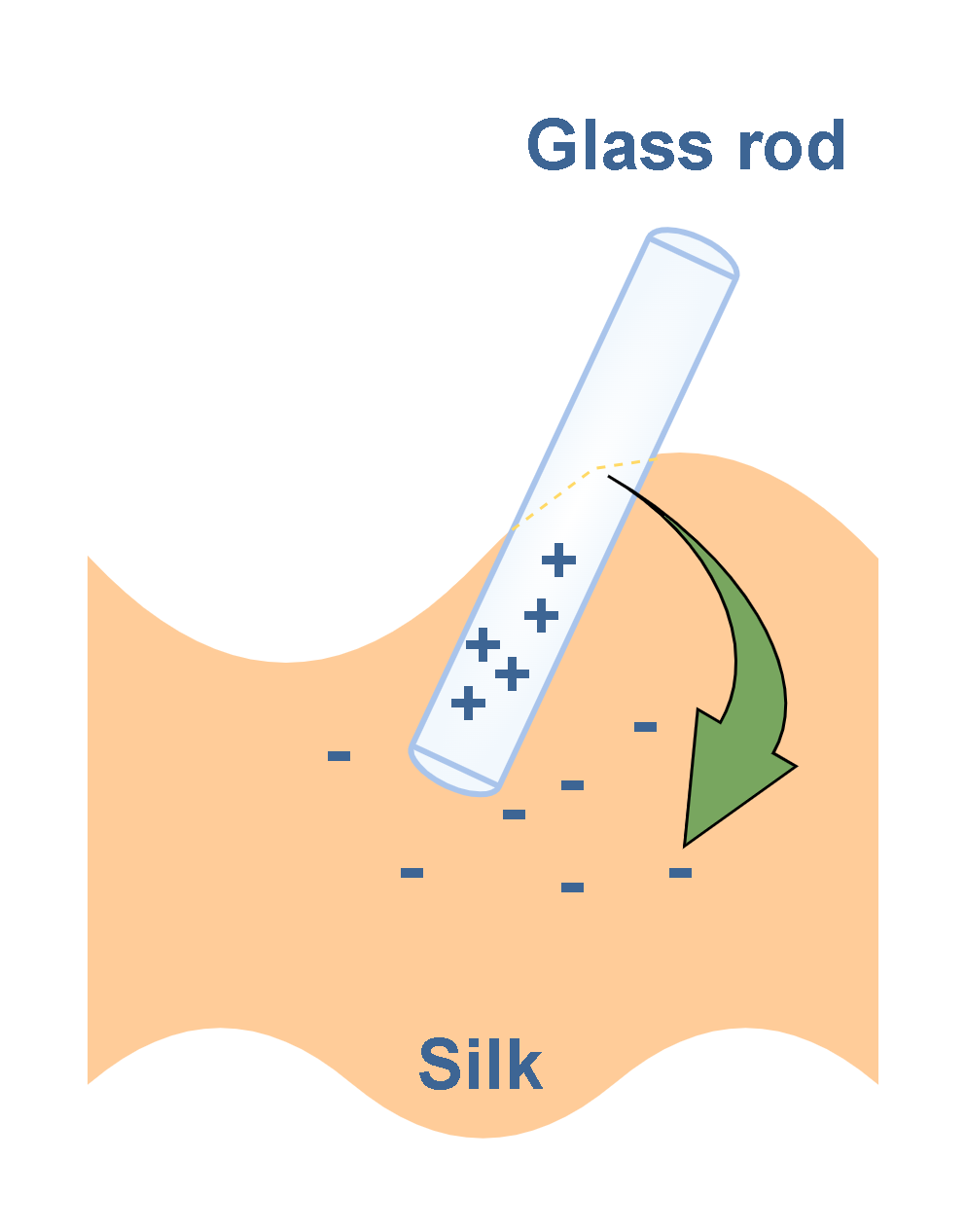
When a glass rod is rubbed with a piece of silk cloth, as in Figure 5, electrons are transferred from the rod to the silk. As a result, the glass rod carries a net positive charge and the silk carries a net negative charge.
The transmission of electric charges between objects, due to rubbing them together, is shown in Figure 6. If there are more protons than electrons, the object will be positively charged, and if there are more electrons than protons, it will be negatively charged. In Figure 6 (a) there are two objects without any physical contact and in the neutral state with equal amounts of positive and negative charges. In Figure 6 (b) and (c) there is physical contact between them which causes the transfer of electrons between the objects. Consequently, we have two charged objects (positively and negatively) instead of neutral ones.
Insulators And Conductors
Substances can be classified in terms of their ability to conduct electric charge. In conductors, electric charges move freely in response to an electric force. All other materials are called insulators.
Glass and rubber are insulators. When such materials are charged by rubbing, only the rubbed area becomes charged, and there is no tendency for the charge to move into other regions of the material. In contrast, materials such as copper, aluminum, and silver are good conductors. When such materials are charged in some small region, the charge readily distributes itself over the entire surface of the material.
Semiconductors are a third class of materials, and their electrical properties are somewhere between those of insulators and those of conductors. Silicon and germanium are well-known semiconductors that are widely used in the fabrication of a variety of electronic devices.
Coulomb´S Law
In 1785 Charles Coulomb experimentally established the fundamental law of electric force between two stationary charged particles. An electric force has the following properties:
- The direction of the electric force is along the line connecting the charges.
- The magnitude of the force F is proportional to the product of the magnitudes of the charges, q1 and q2, of the two particles.
- The magnitude of the force F is inversely proportional to the square of the separation distance r, between the two charges, q1 and q2.
- It is attractive if the charges are of opposite sign and repulsive if the charges have the same sign.
- The force depends on the medium in which the charges are placed.
Based on his observations, Coulomb proposed the following mathematical formula for the vector electric force F between two charges q1 and q2 separated by a distance r as explained in Equation 1.
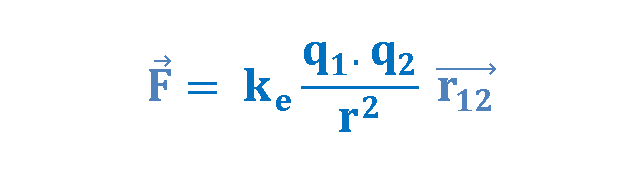
where ke is a constant called the Coulomb constant. The vector electric force F involves both magnitude and direction. In Equation 1 the direction of force is governed by the unit radius vector r12 in the direction from q1 to q2. In the SI system, q1 and q2 are measured in Coulombs (C) and r in meters (m), and the force F should be Newtons (N). The value of the Coulomb constant in Equation 1 depends on the choice of units. From the experiment, we know that the Coulomb constant in SI units has the value explained in Equation 2.
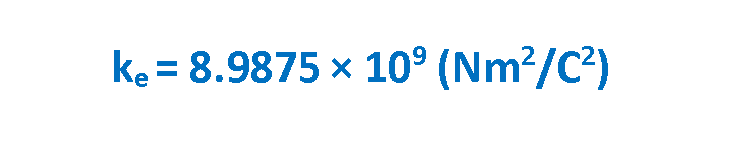
Equation 1, known as Coulomb’s law, applies exactly only to distinct point charges and to spherical distributions of charges, in which case r is the distance between the two centers of charges. If the force formula yields a positive value, charges repel each other. If it yields a negative value, the charges attract each other. Electric forces between stationary and unmoving charges are called electrostatic forces.
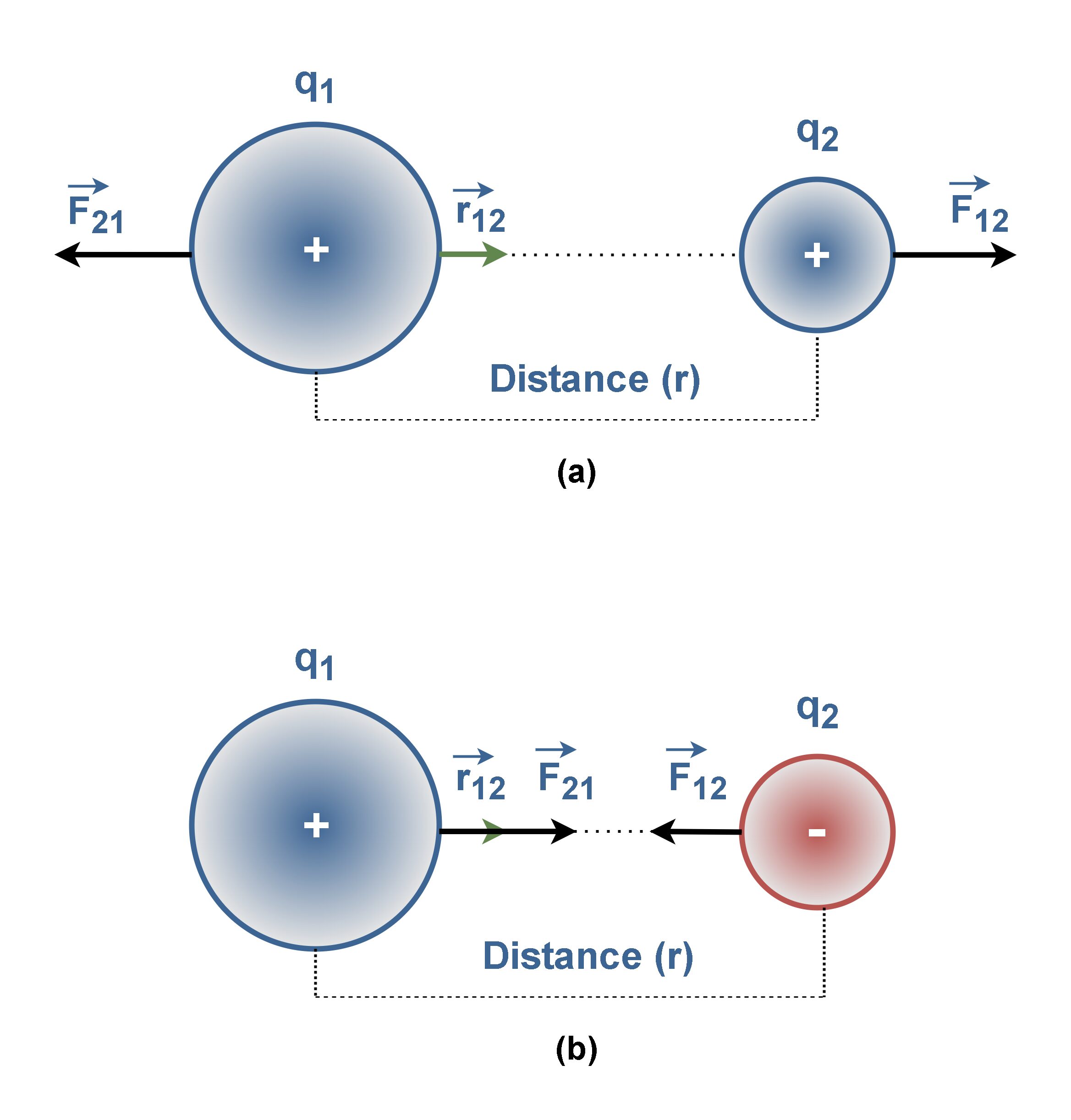
Figure 7 shows the exertion of electric forces, F12 (the force exerted by particle 1 on particle 2) and F21 (the force exerted by particle 2 on particle 1) between similar-sign and dissimilar-sign q1 and q2 charges.
Figure 7(a) shows the electric force of repulsion between two positively (or two negatively) charged particles. Also Figure 7(b) shows the electric force of attraction between two opposite-sign charged particles.
Like other forces, electric forces obey Newton’s third law. Hence, the forces F12 and F21 are always equal in magnitude but opposite in direction, regardless of whether q1 and q2 have the same magnitude or not.
According to Equation 1, if there were two positive equal charges, q, they would repel each other with the force that depends on the product (q × q) as illustrated in Figure 8(a). If each of the charges were reduced by one-half, the repulsion would be reduced to one-quarter of its former value, F/4, as depicted in Figure 8(b).
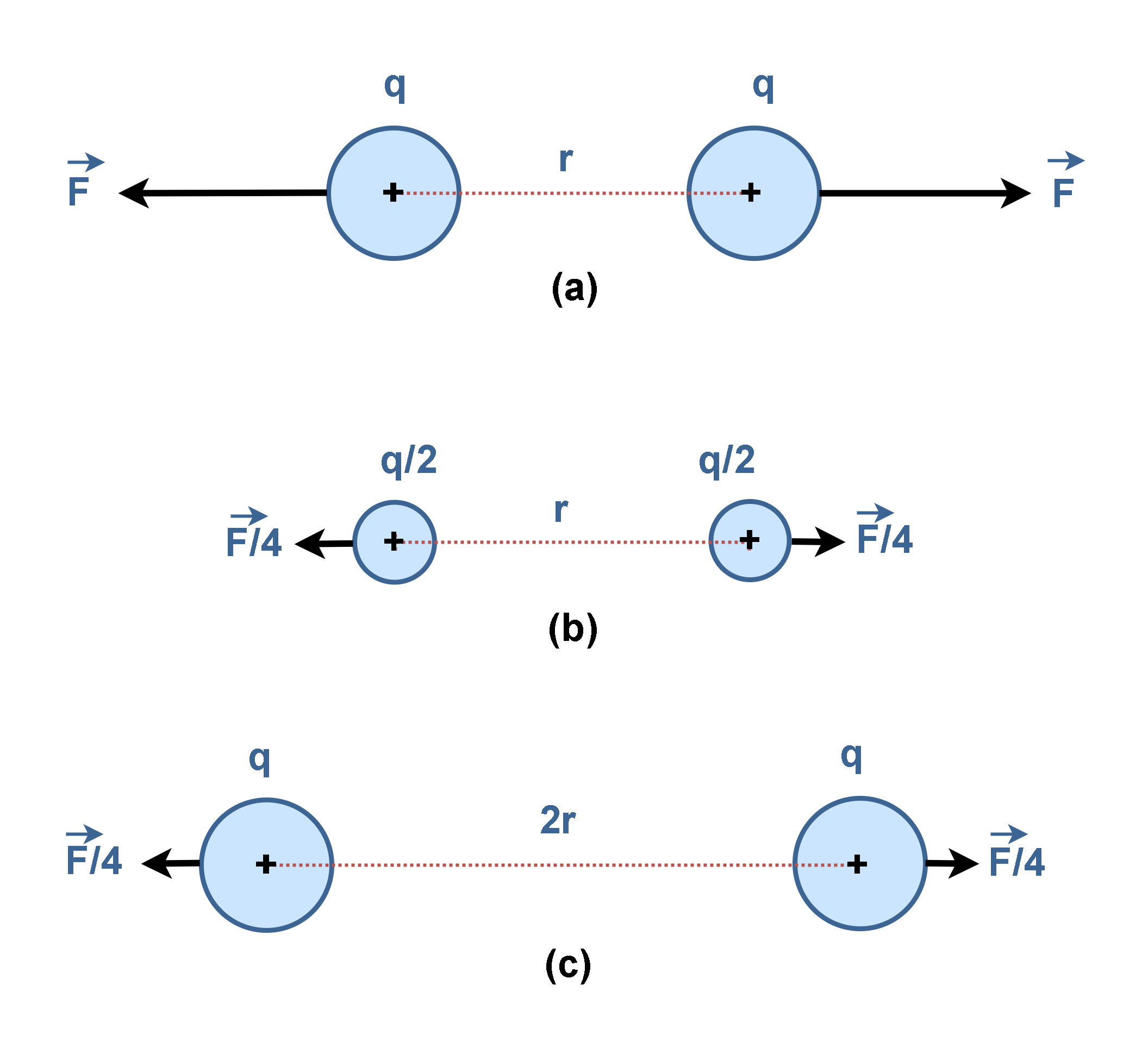
Additionally, if the distance between the two charges is doubled (2 × r), the force becomes weaker, decreasing to one-fourth of the original value (F/4), as depicted in Figure 8(c).
Another expression for the above-mentioned law is related to the definition of the permittivity (ϵ) which is a property of the medium. In the Coulomb’s law, the constant ke can also be written as Equation 3.
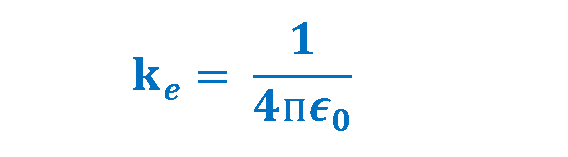
The new constant ϵ0 is called the permittivity of free space and has magnitude, measured in Farads per meter (F/m). Equation 4 defines this quantity.

Thus, the Coulomb’s force law can be rewritten as Equation 5.
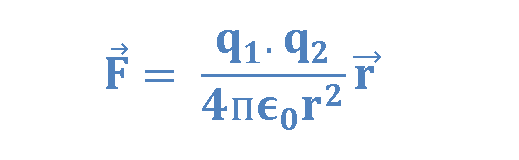
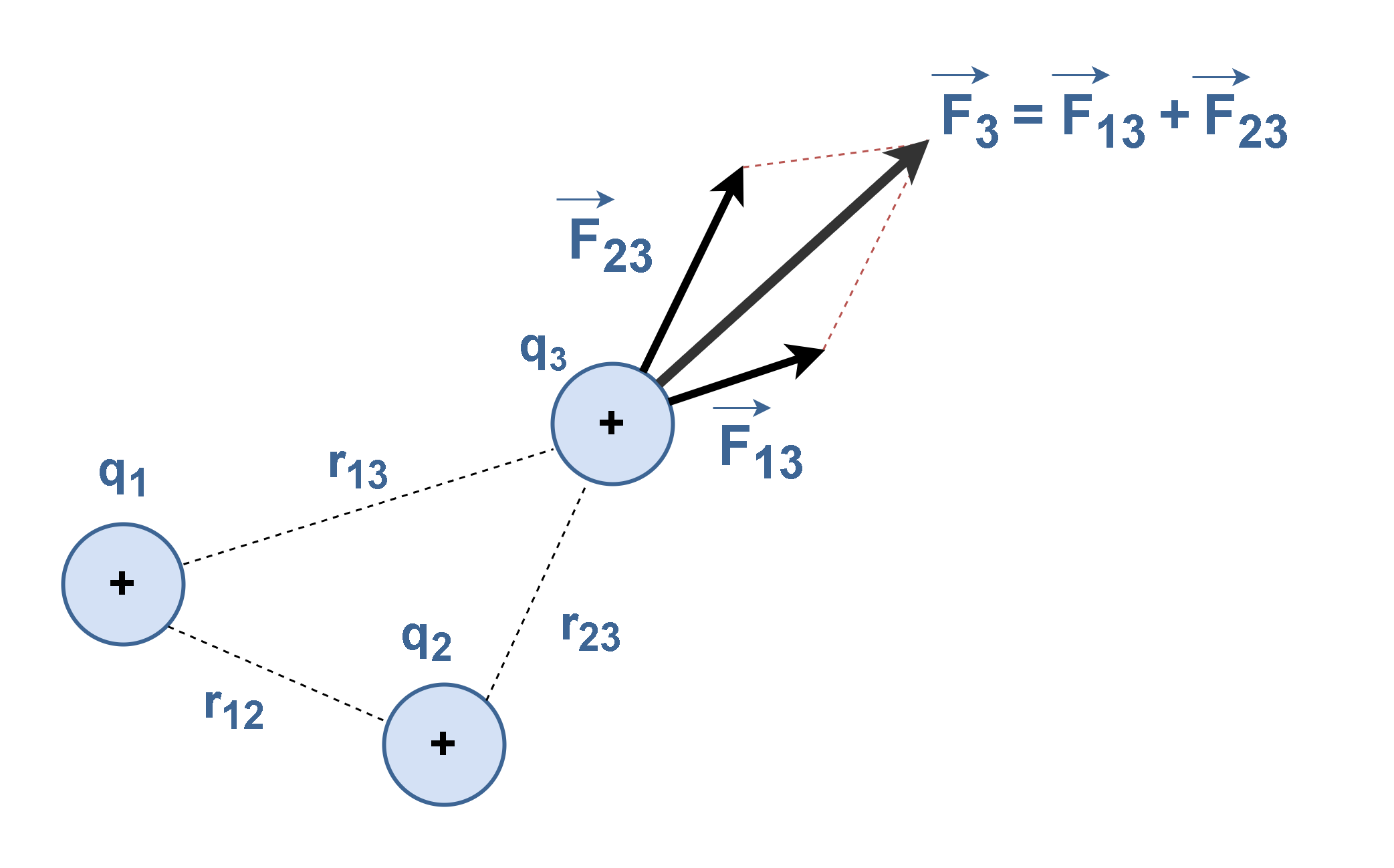
When a number of separate charges act on the charge of interest, each exerts an electric force. These electric forces can all be computed separately, one at a time, and then added as vectors. This is the superposition principle. For example, if there are several charges q1, q2 and q3, the force acting on each charge is the sum of all Coulomb forces acting on the charge from the other charges.
Figure 9 illustrates an example of the final force F3 which is the resultant of the forces exerted on q3 by q1 (F13) and by q2 (F23). Then, F3 is the outcome of vector summation of F13 and F23.
Summary
- Electromagnetism is the science of electric charge and of the forces and fields associated with the charge.
- Electric forces are produced by electric charges either static or in motion.
- Electrostatics describes electric charges at rest.
- Nature’s basic carriers of positive charge are protons, which, along with neutrons, are located in the nucleus of atoms. Some particles, such as a neutrons, have no net charge.
- Then, the smallest subdivision of the amount of charge that a particle can have is the charge of one proton.
- The electron has a charge of the same magnitude but the opposite sign of protons.
- Normally atoms are neutral as the charge of their electrons balances the charge of their core.
- Electrons with negative charges can transfer readily from one type of material to another due to rubbing.
- Objects usually contain equal amounts of positive and negative charge, so they are neutral. Electrical forces between objects arise when those objects have net negative or positive charges.
- Like charges repel one another and unlike charges attract one another.
- Different types of materials are classified as either conductors or insulators on the basis of whether charges can move freely through their constituent matter.
- The SI unit of charge is the coulomb (C).
- Only a very small fraction of the total available charge is transferred between the rod and the rubbing material.
- When using Coulomb’s force law, remember that force F is a vector quantity and must be treated accordingly.
- There are three conditions to be fulfilled for the validity of Coulomb’s law: The charges must have a spherically symmetric distribution, the charges must not overlap, the charges must be stationary.
More tutorials in Electromagnetism
- Electric Conductivity, Resistance and Ohm’s Law
- Steady Current And Current Density
- Electric Displacement and Electrostatic Energy
- Electrostatic Fields In Material Bodies
- The Electric Flux And Gauss’s Law
- Electric Potential In Uniform Fields
- Electric Potential In Nonuniform Fields
- The Electric Field
- The Electric Force