STMicroelectronics is to manufacture cartridges for a multi-sample lab-on-a-chip test system developed by Alifax in Italy. By Nick Flaherty @ eenewseurope.com
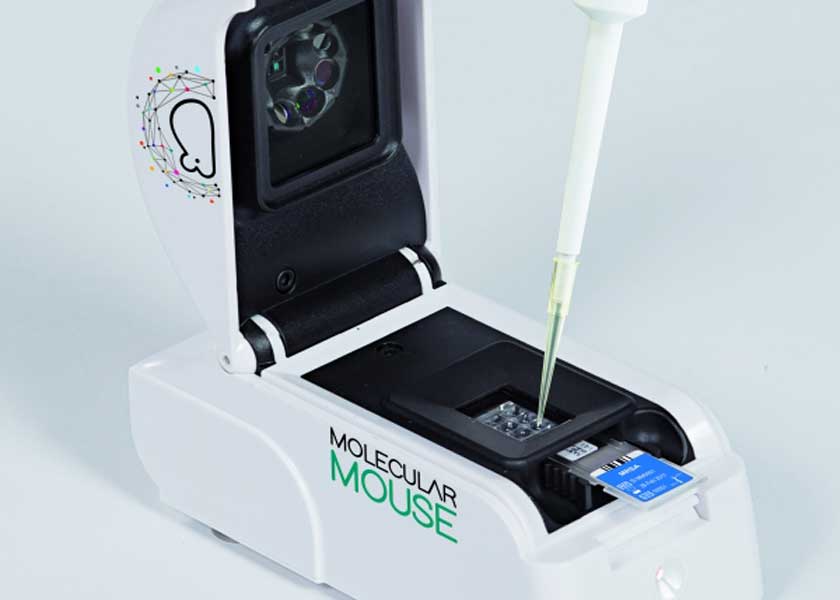
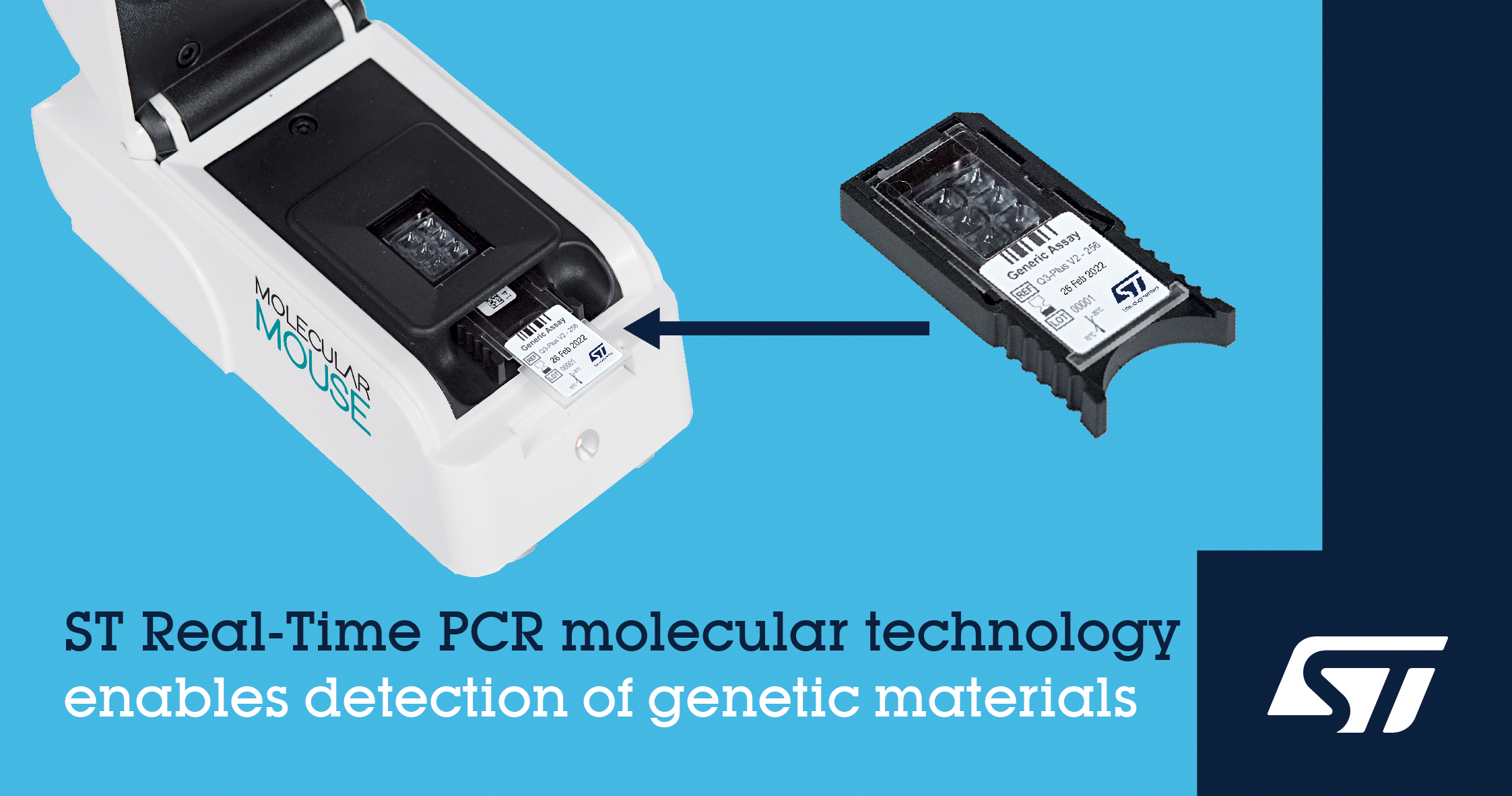
The move extends ST’s manufacturing from packaging the microfluidics lab-on-a-chip device to making the whole cartridge, which measures 46.9mm x 21.9mm x 8.2mm, at its backend plant in Malta. Alifax then adds the chemical reagents for the Polymerase Chain Reactions (PCR) test. Other test cartridges are assembled in a cleanroom.
The cartridge can be used to detect many different types of pathogens, but the main focus is of course Covid-19, with test results in less than an hour. It can also handle multiple samples in a single cartridge, which is a key design feature to boost throughput.
“One crucial lesson from the current global pandemic is the importance of rapid, cost-efficient point-of-care testing that allows immediate remote diagnosis, and then, if necessary, patient isolation,” said Alessandro Cremonesi, Chief Innovation Officer, STMicroelectronics. “ST has been investing in its Real-Time PCR platform convinced that innovative semiconductor-based diagnostic solutions can positively impact our lives.“
“Leveraging our high-volume semiconductor-manufacturing technology and long-term leadership in microfluidics, we’ve developed a rapidly customizable, highly flexible cartridge and instrumentation platform that delivers rapid and precise point-of-care diagnostic results, which Alifax has used to respond almost immediately to present pandemic and future diagnostic needs,” added Benedetto Vigna, President Analog MEMS, and Sensors Group, STMicroelectronics.
“Building on our passion for research and excellence in innovation, Alifax has established its strength in hematology and bacteriology for diagnostic purposes. By working closely with ST and combining its foundational microfluidic and other technology in the Molecular Mouse and our pathogen-specific assays, starting from a COVID-19 test, we’re ready to contribute to rapidly diagnosing, isolating, and ultimately stopping the spread of pathogens,” said Paolo Galiano, President of Alifax.
Alifax had been developing 4-5 assay panels for sepsis, and one assay to detect Zika, Dengue, Chikungunya when the pandemic began and pivoted to develop the Covid-19 assays. The sepsis and Zika, Dengue, and Chikungunya assays will follow soon.