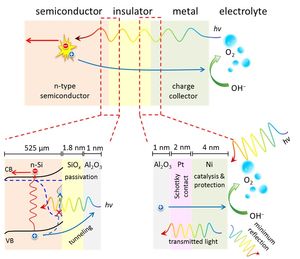
Using solar energy to split water provides an efficient way for large scale renewable energy conversion and storage. A group of researchers from TUDelft and AMOLF have successfully developed an efficient and stable photo-electrode that could improve water splitting with solar energy.
This photoelectrode absorbs light and directly decomposes water into hydrogen and oxygen. In addition to the efficiency, the system is also cheap because of using silicon wafers as the light absorbing material.
The Process
Photoelectrochemical (PEC) splitting of water is a direct conversion of solar to chemical energy to produce renewable and clean fuel. The hydrogen, for example, can be used directly in fuel cells, or combined with other molecules to create durable materials.
Together with colleagues from AMOLF (Amsterdam), we have engineered a photo-electrode, a material that absorbs light and directly splits water, that has a very high efficiency and over 200 hours of stability’, says Wilson Smith, Associate Professor in the Department of Chemical Engineering at TU Delft. ‘This is remarkable in a field where people normally show only a few hours of stability. We use silicon wafers as the light absorbing material, so the photoelectrode is also very cheap.
Researchers had also designed a new insulator layer to stabilize the semiconductor (Si) photo-electrode, while keeping the high efficiency of water splitting by using two metals. This approach known as making a metal-insulator-semiconductor (MIS) junction. It is a simple system that combines the stability and catalysis bottlenecks in photoelectrochemical water splitting.
For more information, the researchers had published this research in Nature Communications.